Zebrafish - Genetic, Developmental and Behavioral Model

1. RNA editing in zebrafish - The involvement of epigenetic RNA editing in neurobehavioral processes, embryonic development and cell physiology.
2. Zebrafish model for depression and anxiety -
- Deciphering the genetic code of depression and anxiety.
- Studying the effect of known antidepressants on the etiology of depression and the neurocircuits underlying it.
- Large scale screening for new medication for treating depressive behaviors.
Group leader
Dr. Limor Ziv-Strasser
Molecular Neuroscience

Using molecular techniques and animal models to analyze genetic and epigenetic processes affecting learning and behavior.
Research Programs
1. Glutamate receptors and RNA editing in Alzheimer's disease (in collaboration with Dr. Anat Biegon, Sagol Center for Neuroscience)
2. Lentivirus-mediated gene silencing in vitro: the effect of decreased expression of RNA editing enzymes on glutamate neurotransmission and neuronal cell proliferation
3. Lentivirus-mediated gene silencing in vivo: the effect of decreased expression of RNA editing enzymes 0n rodent behavior.
Group Leader
Inna Gaisler-Salomon, PhD
Tumor Stem Cell Biology
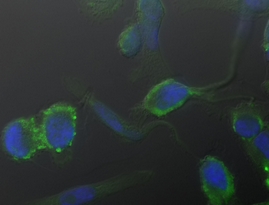
Applying stem cell concepts to identify novel therapeutic targets in cancer. To this aim we established specialized culture conditions to enrich for precursor cells in melanoma. Gene expression studies as well as functional assays revealed a critical role for the non canonical wnt pathway in melanoma. In addition, we use induced pluripotent stem cells obtained from patients carrying genetic mutations to model genetic diseases. This system will be used to scan novel therapies in tissues such as muscle and neurons which are hard to access. Finally, we are studying the association of genetic variation in regulatory elements to cancer and neuropsychiatric diseases.
Group Leader
Gilmor I. Keshet, Ph.D.
Team
Liraz Shenhav, M.Sc. - Research assistant
Hadar Fudem, B.Sc. - (MSc Student)
The Research Center of Leukemia and Childhood Malignancies
Cancer is the leading cause of death from disease in children. Leukemias and hematopoietic malignancies are the most common types of cancer in children. Although mortality rates for these malignancies have declined significantly, the cure involves intensive and toxic chemotherapy. Our ultimate goal is to cure all children with leukemia and cancer with personalized adjustment of therapy to the severity of the disease and to the molecular genetic abnormalities in the cancer cells. A prerequisite for the achievement of this goal is better understanding of how children get leukemia and what are the differences between normal and cancer cells. This can be realistically achieved by translational research utilizing modern sophisticated genetic and biochemical tools.
Head
Prof. Shai Izraeli
Lab Team:
Yehudit Birger PhD - senior scientist, lab manager
Inna Muler - Technician
Liat Goldberg PhD - Postdoctoral fellow
PhD Students
Asher Castiel
Ahuvit David
Ithamar Ganmore
Nir Gefen
Liron Frishman-Levi
Libi Hertzberg
Gil Smooha
Research Laboratory Pediatric Oncology and Stem Cell Transplantation
The driving force and the subjects for research of the lab come from the Pediatric Hemato-Oncology Department and Clinics. The research ideas stem from the sick child's bed, the resistant leukemias, metastatic sarcomas, and incurable brain tumors. The fruits of such a research may very soon find their application in the clinics as the research doctors make their daily rounds in the ward treating their cancer patients.
Topics of research
1.The anti tumoral molecular mechanisms of "new drugs" with regard to pediatric leukemias, Neuroblastomas, and brain tumors cell lines and primary cells.
2. Molecular characterization of stem cells from various sources: bone marrow, cord blood, peripheral blood and fetal liver.
3. Identification of specific mutations in Glioblastomas and the preparation of patient specific probes for the early detection of minimal residual disease.
- Methylation status of MGMT in pediatric high grade glioma stem cells.
- The effect of "new drugs" on high grade glioma stem cells.
Head:
Professor Amos Toren
Lab manager, senior scientist:
Tamar Fisher PhD.
MSc Students:
Tali Harush
Sarit Eder
Researching physicians
Dalia Waldman MD
Hana Golan MD
Bella Bielorai MD
Michal Yalon MD
Gal Goldstein MD
Professor Chaim Kaplisky
Molecular Diagnostic Center (MDC)

Our Mission
To develop predictive biomarkers for various cancer therapies that will play a critical role in the efforts to best match patients to the most effective treatments. It will foster discoveries that will enable physicians to determine the specific genetic and molecular abnormalities in each patient's cancer and then prescribe the appropriate therapy.
The MDC primary work is
-
Laboratory discoveries with the greatest potential to lead to innovative diagnostic mechanisms to identify key targets in a patient's tumor and predictive and prognostic markers
-
Provide new diagnostic tests for established predictive and prognostic markers including mutations for other candidate genes predictive for treatments efficacy:
· KRAS mutations predictive for cetuximab treatment for metastatic colorectal patients
· BRAF - mutations predictive for Erbitux / Panitumumab treatment for colorectal patients, RAF inhibitors for melanoma patients, and Papillary Thyroid Carcinoma for the most appropriate treatment.
· EGFR - mutations predictive for Tarceva or Iressa for non small cell lung carcinoma.
· C-Kit and PDGFRA mutations for Imatinib and Sunitinib predictive in Gastrointestinal Stromal Tumors (GIST).
· PTEN
· PIK3CA
· HER2
-
Pharmacogenetics
· CYP2D6 polymorphism - Tamoxifen treatment efficacy
· UGT1A1 polymorphism - CPT-11 treatment toxicity
· DPD expression- 5FU toxicity
The MDC at Tel Hasomer provides DNA based testing for diagnosis of variety of diseases. All tests are performed on paraffin blocks, using clinical protocols, assuring accurate results. All reports are detailed.
Results are reported directly to referring physician to discuss interpretation.
Technology
Sequenom massarray iPLEX platform
One of the methods to translate DNA information into complete identification of bio-markers. The technology can provide genetic analysis for biomedical research and molecular medicine applications.
We can identify single nucleotide polymorphism (SNP) genotype, base on locus specific poly chain reaction (PCR), followed by single base DNA extension. In the next step by using the Sequenom, the mass of the extended primer is determined. The different in the weight, point out on changes at the polymorphic site. By using the Sequenom software, we can get translation of the mass to genotype.
The DNA analysis is reliable and specific and can be done on complex biological samples like tissue culture and tumors, and on simple biological samples like blood.
Real Time PCR
By using real time PCR we can detect and quantify specific relatively short DNA sequence particularly in genetic abnormality as SNP's. We will use the technique at MDC to look for changes in gene expression over time and for response of tissue or cell culture before and after therapy.
Length analysis
Allele-specific PCR and specific primers can discriminate small changes in DNA length. Identification of insertion or deletion of small DNA fragments as well as SNP in the DNA, base on that length analysis and size separation, can lead to detection of genetic changes in the DNA.
Fluorescent in situ hybridization-FISH
FISH is sensitive diagnostic tool for detection and localization of a specific DNA sequence on chromosome. By using isolated tagged fragment of DNA that can incorporate directly to the sequence of interest, we can detect the presents or absence of DNA sequence in DNA from cancer patients.
Immuno-staining
Staining of tissue sections after fixation to give colored product will provide us with diagnosis information about present of molecular markers of specific types of cancers.
With that method we will be able to learn about present of proteins, expression and distribution of them in the tissue section.
Professional stuff
Dr. Raanan Berger, M.D., Ph.D
Dr. Rinat Yacobi, Ph.D
Flow cytometry and cell sorting

1. Cell-to-cell transfer of oncogenic molecules and miRNAs from cancer cells to lymphocytes: Our group has pioneered this field and our present aims are as follows:
(i) systematically determine the role of this mode of cellular communication in cancer immuno-surveillance and -editing; (ii) to unravel the full spectrum of proteins and small regulatory RNAs that are transported at the immunological synapse; and (iii) to resolve the molecular mechanism(s) that facilitate this unique cell-to-cell signal transfer at the immunological synapse.
2. Translational Research in the field of regulatory T (Treg) cells: (i) develop Good Manufacturingg Practice (GMP) grade protocols to generate Tregs ex vivo for "patient tailored" immunotherapy using novel technologies; (ii) to examine in "humanized" NOG (NOD/Shi-scid/IL-2Rγnull) mice the efficacy of ex vivo induced human Treg cells in preventing immune mediated target organ damage.
Group leader
Itamar Goldstein MD
Team:
Helly Vernitsky, M.Sc. (PhD. student): Projects 1 & 2.
Meital Nagar, M.Sc. (PhD. student): Project 2.
Nir Rainy, M.Sc. (PhD. student): Project 1.
The Ella Institute for Treatment and Research of Melanoma
The Ella Institute offers a wide range of the most advanced oncology treatment and diagnostic techniques in melanoma and skin cancer as well as state of the art research laboratories in immunotherapy of cancer.
The institute is headed by Prof. Jacob Schachter- Deputy Director Oncology Department at the Sheba Medical Center, Senior lecturer at the Tel Aviv University, Head of the Israeli forum of Melanoma. The Institute is part of the Oncology Division, Prof Raphael Catane - chairman, and The Cancer Research Center, Prof Gidi Rechavi - Chairman, at the Sheba Medical Center, Tel Hashomer, Israel.
Treatment of Metastatic Melanoma: The Ella Institute offers a new experimental treatment for patients with metastatic melanoma. This innovative treatment was developed in the United States at the National Cancer Institute (NCI) and is based on adoptive immunotherapy of anti-cancer lymphocytes. The Sheba Medical Center is the only hospital out of the US which offers this innovative treatment, and applied it already successfully in advanced melanoma patients, which failed all other treatments. The treatment is based on amplifying and adoptive transfer of the patient's own white blood cells (TIL - tumor infiltrating lymphocytes), which can react against the tumor tissue, together with myeloablative chemotherapy and interluikin 2. Clinical results of the treatment in Sheba were published recently.
The Ella Institute also offers advanced bio-chemo treatment such as High dose IL2 and Interferone, combined with chemotherapy to metastatic melanoma patients.
For melanoma patients with high risk for recurrence, the Ella Institute offers advanced adjuvant treatments such as high dose interferon alpha and other modalities.
A world expert scientific team is leading research and development programs for anti-cancer modalities in state of the art research laboratories, as well as GTP (good tissue culture practice) laboratory, equipped for translational and clinical production of cellular therapies.
The Ella Institute WebSite
Cancer Epigenetics Laboratory
Epigenetics refers to heritable patterns of gene expression mediated by changes in the packaging of the genetic material rather then changes in the DNA sequence itself. In recent years, it has been increasingly recognized that aberrant epigenetic mechanisms play major roles in the carcinogenic process. These mechanisms act on the level of DNA - via DNA methylation or on the chromatin level - via various histone modifications such as those mediated by the repressive Polycomb complex. The aberrant action of these mechanisms may cause down regulation of tumor suppressive pathways or up regulation of oncogenic pathways contributing to tumorgenesis.
Our aim is to characterize genome wide changes of DNA methylation and Polycomb group occupation using next generation sequencing based technologies in various in vivo and in vitro cancer models:
1) Primary breast tumors of various subtypes and normal breast tissue,
2) In vitro transformation models of human mammary epithelial cells.
3) Transformation models of prostate epithelial cells harbouring the T/ERG translocation found in high percentage in prostate carcinomas.
We expect these studies will provide a characteristic "epigenomic landscape" providing a basis for understanding the relative contributions of these mechanisms to the cancer phenotype and the interrelations between them.
Head
Einav Nili Gal-Yam MD, PhD
Team:
Adi Zundelevich, PhD.
Laboratory of Genomic Applications in Surgical Oncology
Hereditary and non-hereditary breast cancer progression and maintenance of genome stability mechanisms.
Breast carcinoma is one of the most common neoplasms in women and is a leading cause of cancer related deaths worldwide. Until recently the dogma of breast cancer development involved a sequential progression from defined clinical and pathological stages starting with atypical ductal hyperplasia (ADH), progressing to in situ ductal carcinoma (DCIS), then invasive ductal carcinomas (IDC) and culminating in metastatic Disease. Utilizing high throughput molecular profiling techniques, the simplicity of that model is being challenged and it has been suggested that low grade DCIS will develop into low grade IDC while high grade DCIS will develop into aggressive IDC. Due to improved diagnostic tools, there are increased number of patients diagnosed with pre-invasive breast lesions. Although only 30% of pre-malignant lesions identified progress into invasive tumors, all diagnosed women are subjected to similar aggressive treatment of radiation therapy and adjuvant hormonal therapy. Our research focuses on the identification of genetic and epigenetic molecular markers that can distinguish between potentially aggressive to non-aggressive DCIS tumors.
Hereditary breast cancer is the most common hereditary cancer syndrome in Israel. Two genes are associated with hereditary breast cancer, BRCA1 and BRCA2. Mutations in these genes predispose women to early onset breast and ovarian cancer. While human genetics makes it clear that these genes function as tumor suppressors, it is still unclear how these proteins function. The research in our lab focuses on gaining mechanistic insights into the multiple cellular roles of BRCA1 in guarding the genome and suppressing cancer development. We found that BRCA1 is required for G2/M cell cycle checkpoint arrest in order to prevent unscheduled mitotic entry following DNA damage. As BRCA1 is an E3 ubiquitin ligase we are actively pursuing studies how BRCA1 ubiquitin ligase enzymatic activity is related to its role in cell cycle checkpoint control and maintenance of genomic stability. Current work also addresses the mechanisms of chromatin structure regulation in the context of the DNA damage response.
On-going research projects also include the investigation of micronutrients as vitamin A and D and their nuclear receptors in the prevention and treatment of human cancers. Both vitamin A and vitamin D trigger growth inhibition and differentiation of tumor cells. Their mechanism of action through transcriptional and chromatin structure regulation and their interaction with tumor suppressors including BRCA1 is being studied with the overall objective of developing new preventive and therapeutic strategies for cancer patients.
Ronit Yarden, Ph.D
Laboratory Head,
WebSite of Laboratory of Genomic Applications in Surgical Oncology
Lung Cancer Research Laboratory
Topics of research
1. Analysis of genetic and epigenetic changes in lung cancer.
The project is funded by the by the FAMRI Centers of Excellence. As a part of the project we are collaborating with a team at Johns Hopkins University.
2. Pathogenesis of malignant pleural effusion associated with human lung adenocarcinoma.
Screening for structural and functional abnormalities of lung tumor blood vessels and the expression of receptor tyrosine kinase ligands that may be involved in tumor vascular hyperpermeability and vascular leakage
3. Development of an orthotopic mouse model to study the biology and therapy of lung cancer, including the biology and treatment of malignant pleural effusion.
Head:
Dr Amir Onn, MD
Lab manager:
Dr Maya Damianovich, MD, PhD
Team:
Goni Hout Siloni
Laboratory of Endocrine Oncology
Cancer development is tightly associated with endocrine activity. The research in our laboratory is dedicated to the investigation of endocrinological aspects of breast cancer, as well as other malignant diseases. We combine laboratory, genetic and clinical studies and conduct our studies in collaboration with the Endocrinology Institute of the Sheba Medical Center.
Recent projects
Identification of klotho as a tumor suppressor in breast and pancreatic cancers: The klotho protein plays an important role in various physiologic activities and its deletion in mice results in pre-mature aging. We identified down-regulation of klotho in breast and pancreatic cancers and discovered the role of klotho as a modulator of signaling pathways in these tumors. Moreover, we found that restoring its expression can inhibit growth of cancer cells both in vivo and in vitro.
Klotho as a risk modifier for breast cancer among BRCA1 and 2 mutations carriers: A functional variant of klotho is associated with aging and other human diseases. We identified a strong association between the presence of the variant and the cancer risk among BRCA1 and 2 mutations carriers. This important finding may provide a novel tool for predicting cancer risk among these women.
Diabetes mellitus and breast cancer: We conducted a retrospective analysis and identified an association between diabetes mellitus and adverse prognostic factors in breast cancer. We also conducted a meta-analysis of published studies and identified significant correlation between diabetes mellitus and breast cancer risk. Following these discoveries we are currently exploring a role the hormone GLP-1 and of the novel anti-diabetic drug exendin-4 in the development of breast cancer.
Ido Wolf, MD; Tami Rubinek, PhD
Lab team:
Lilach Abramovitz, PhD
Hagai Ligumsky
Shiri Shahamon
Ira Lojkin
The Laboratory for Molecular Cell Biology
The biology, biochemistry and clinical applications of small non-coding RNAs. Small RNAs molecules play a major role in cell proliferation, differentiation and determination of cell fate and where also found to be involved in many types of cancer.
1) Small double stranded synthetic RNA molecules (siRNAs) may serve as potentially specific target drugs. We are developing siRNA molecules that use new approach to deliver into cells and longer silencing effect.
2) Psoriasis is a complex disease at the cellular, genomic and genetic level. The importance of miRNAs in human skin development was already shown. Study the involvement of miRNAs in the pathogenesis of psoriasis. We have found few miRNAs that were expressed differentially, between psoriasis to normal skin (figure 1). We are further exploring the involvement of these miRNAs in the development of the disease.
3) Malignant melanoma is devestating skin cancer with a constantly increasing incidence worldwide. In recent years, it is becoming clear that aberrant expression of miRNAs has a role in cancerous transformation and progression. Our aim is studying the role of miRNAs in the pathogenesis of malignant melanoma. Should this be the case, our work will enlight potential novel therapeutic targets in melanoma.
Professor Sidi Yechezkel MD
Avni Dror PhD
Raya Leibowitz-Amit MD, PhD
Lab team:
Lerman Gali - Ph.D student
Bonen Hamutal - Ph.D student
Mizrahi Adi - Ph.D student
Zehvi Liron - Ph.D student
Gembitz Anat - M.Sc. student
Sergeyev Victoria - technician